NeuroStar Transcranial Magnetic Stimulation
ABOUT NEUROSTAR TMS
 NeuroStar utilizes transcranial magnetic stimulation (TMS) to stimulate specific regions of the brain that are underactive in individuals experiencing depression. Unlike electroconvulsive therapy (ECT), TMS is a different, noninvasive approach.
NeuroStar utilizes transcranial magnetic stimulation (TMS) to stimulate specific regions of the brain that are underactive in individuals experiencing depression. Unlike electroconvulsive therapy (ECT), TMS is a different, noninvasive approach.
Although the precise cause of depression remains unclear, prevailing research suggests that it may result from an imbalance in neurotransmitters—chemical messengers that facilitate communication between brain cells.
What is NeuroStar Advanced Therapy (TMS)?
NeuroStar employs precisely targeted magnetic pulses, comparable in strength to an MRI, to activate underperforming areas of the brain involved in mood regulation. When these regions are not functioning optimally, depression may develop. Revitalizing these areas can lead to significant, lasting improvements, offering many individuals long-term relief from depression.
HOW IT WORKS
NeuroStar Advanced Therapy is a simple and convenient treatment:
- Sessions take place at your NeuroStar provider’s office.
- You can resume regular activities immediately afterward.
- Treatment is noninvasive, and you remain fully awake throughout.
- It does not negatively impact memory or sleep patterns.
- Most major insurance plans, including Medicare and Tricare, cover the treatment.
Here’s what you can expect from a NeuroStar Advanced Therapy (TMS) session:
Before Treatment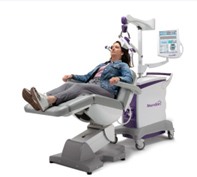 You’ll recline comfortably in the treatment chair as a curved magnetic coil is positioned on your head.
You’ll recline comfortably in the treatment chair as a curved magnetic coil is positioned on your head.
During Treatment
You may hear a clicking sound and feel a tapping sensation as the magnetic pulses target specific brain regions associated with depression. The patented Contact Sensing technology ensures precise delivery of the prescribed dose during each session.
After Treatment
Patients remain alert and can drive themselves home or return to daily activities immediately. A standard treatment plan includes 36 sessions, each lasting 19–37 minutes, depending on your doctor’s recommendation. Completing the full course maximizes the potential for long-term relief.
FAQs
Q: What is NeuroStar Transcranial Magnetic Stimulation (TMS)?
NeuroStar TMS is a drug-free, noninvasive treatment that uses targeted magnetic fields to stimulate underactive brain areas, helping to alleviate symptoms of depression. It is an effective option for individuals who have not achieved relief with antidepressants.
Q: How does NeuroStar TMS work?
Focused magnetic pulses activate underactive regions of the brain responsible for mood regulation. By “waking up” these areas, TMS helps achieve lasting improvements in depressive symptoms.
Q: How long is NeuroStar TMS treatment?
A typical treatment plan involves 36 sessions, each lasting 19–37 minutes. Your doctor will tailor the schedule to your needs. Completing the entire course ensures the best chance for long-lasting results.
Q: Is NeuroStar TMS Therapy covered by my insurance?
NeuroStar TMS is covered by most major insurance providers, including Medicare and Tricare, making it accessible to over 300 million individuals with insurance coverage.
Q: Is TMS suitable for patients who cannot tolerate antidepressant side effects?
NeuroStar TMS offers an effective alternative without the systemic side effects of medication. The most common side effect is mild to moderate discomfort at the treatment site, which typically subsides after the first week.
Q: How does NeuroStar TMS differ from other magnetic therapies?
Unlike low-intensity, static magnetic field products, TMS uses high-intensity pulsed magnetic fields that effectively activate brain cells. This targeted activation is critical for its therapeutic impact.
At Serenity Health Center in Florida, we’re here to help you rediscover joy and live life to the fullest. If you’ve been struggling with depression and haven’t found relief with traditional treatments, NeuroStar Advanced Therapy (TMS) could be the solution you need. To book your appointment, please call (352) 241-9282.
INDICATION STATEMENT
The NeuroStar Advanced Therapy System is indicated for the treatment of depressive episodes and for decreasing anxiety symptoms for those who may exhibit comorbid anxiety symptoms in adult patients suffering from Major Depressive Disorder (MDD) and who failed to achieve satisfactory improvement from previous antidepressant medication treatment in the current episode.
The NeuroStar Advanced Therapy system is intended to be used as an adjunct for the treatment of adult patients suffering from Obsessive-Compulsive Disorder (OCD).
NeuroStar Advanced Therapy is only available by prescription. A doctor can help decide if NeuroStar Advanced Therapy is right for you. Patients’ results may vary.
The most common side effect is pain or discomfort at or near the treatment site. These events are transient; they occur during the TMS treatment course and do not occur for most patients after the first week of treatment. There is a rare risk of seizure associated with the use of TMS therapy (<0.1% per patient).
Visit neurostar.com for full safety and prescribing information.
CLINICAL & ACADEMIC REFERENCES
Carpenter LL, et al. (2012). Transcranial Magnetic Stimulation (TMS) for Major Depression: A Multisite, Naturalistic, Observational Study of Acute Treatment Outcomes in Clinical Practice. Depression and Anxiety, 29(7):587-596. www.ncbi.nlm.nih.gov/pubmed/22689344
George MS, et al. (2010). Daily Left Prefrontal Transcranial Magnetic Stimulation Therapy for Major Depressive Disorder: A Sham-Controlled Randomized Trial. Arch Gen Psychiatry, 67(5):507-516. www.ncbi.nlm.nih.gov/pubmed/20439832
Dunner DL, et al. (2014). A Multisite, Naturalistic, Observational Study of Transcranial Magnetic Stimulation (TMS) for Patients with Pharmacoresistant Major Depressive Disorder: Durability of Benefit Over a 1-Year Follow-Up Period. J Clin Psychiatry. 75(12):1394-401. www.ncbi.nlm.nih.gov/pubmed/25271871
O’Reardon JP, et al. (2007). Efficacy and Safety of Transcranial Magnetic Stimulation in the Acute Treatment of Major Depression: A Multisite Randomized Controlled Trial. Biol Psychiatry, 62(11):1208-1216. www.ncbi.nlm.nih.gov/pubmed/17573044




